
Zebrafish: Dorsal view
Recent research has unveiled a promising avenue for heart repair by harnessing a protein known as HMGA1, derived from zebrafish, a species renowned for its remarkable regenerative abilities. This protein has demonstrated the capacity to activate dormant repair genes in mammalian hearts, offering potential breakthroughs in treating heart failure.
Understanding HMGA1 and Its Role
HMGA1 belongs to the High Mobility Group A (HMGA) family of proteins, which are architectural chromatin proteins. These proteins possess AT-hooks that enable them to bind to the minor groove of AT-rich regions in DNA, facilitating chromatin remodeling and influencing gene expression. In simpler terms, Hmga1 acts like a molecular locksmith, unlocking tightly packed DNA regions to allow specific genes to become active.
In zebrafish, HMGA1 is instrumental during embryonic development, promoting cell growth and differentiation. Its role diminishes in adult stages but reactivates upon cardiac injury, leading to heart regeneration. This regenerative capacity is absent in adult mammals, including humans, where heart muscle cells (cardiomyocytes) have a limited ability to proliferate after injury.
Key Insight
Think of HMGA1 as a molecular locksmith - it unlocks tightly packed DNA regions to allow specific repair genes to become active, enabling the heart to heal itself.
Mechanism of Action: Loosening Chromatin for Gene Activation
Chromatin, the complex of DNA and proteins within the nucleus, can exist in a tightly packed (heterochromatin) or loosely packed (euchromatin) state. The packing state determines gene accessibility: tightly packed chromatin silences genes, while loosely packed chromatin allows gene activation. HMGA1 influences this dynamic by binding to DNA and inducing structural changes that loosen the chromatin, thereby activating genes essential for cell proliferation and repair.
Simple Analogy
Think of it like this: a garden hose, when tightly coiled, restricts water flow (gene expression); when uncoiled, water flows freely. HMGA1 functions to uncoil the hose, ensuring that the necessary genes are accessible for heart repair processes.

Introduction - when the earth hums, we listen
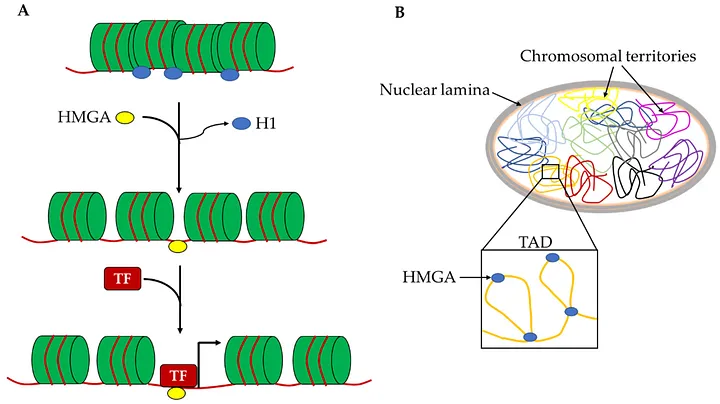
Model of HMGA1 loosening chromatin for gene expression
Experimental Findings in Mouse Models
Researchers applied HMGA1 to damaged mouse hearts to assess its therapeutic potential. The results were remarkable: Hmga1 stimulated heart muscle cells to divide and grow, significantly improving heart function. Notably, this regenerative activity was confined to the injured areas, with no adverse effects such as excessive growth or heart enlargement observed.
This specificity suggests that HMGA1 activation is finely tuned to respond to injury signals, promoting repair precisely where needed without affecting healthy tissue. Such targeted action minimizes the risk of unintended consequences, a critical consideration in developing safe therapeutic interventions.
Improved Function
Significant improvement in heart function after HMGA1 treatment
Targeted Action
Regenerative activity confined only to injured areas
No Side Effects
No adverse effects or excessive growth observed
Implications for Human Heart Repair
Unlike zebrafish, human hearts do not naturally regenerate damaged tissue effectively. After a heart attack, human cardiomyocytes lack the signals to induce repair, leading to scar formation and compromised heart function. However, humans possess the HMGA1 gene, which is active during embryonic development but becomes dormant in adulthood.
The presence of the HMGA1 gene in humans opens the possibility of reactivating it to promote heart repair. By inducing HMGA1 expression in adult human hearts, it may be feasible to unlock the dormant regenerative pathways, akin to the natural processes observed in zebrafish. This strategy could revolutionize treatments for heart failure, shifting from managing symptoms to actively repairing damaged heart tissue.
Safety and Specificity Considerations
The mouse model studies have shown that HMGA1-induced regeneration occurs exclusively in damaged heart tissue, with no adverse effects in healthy areas. This precision is crucial for therapeutic applications, as it reduces the risk of complications such as uncontrolled cell growth or tumor formation. The absence of adverse effects in preclinical models provides a strong foundation for advancing this research toward human clinical trials.
Future Directions and Challenges
While the findings are promising, several challenges remain before HMGA1-based therapies can be applied to humans. Key areas for future research include:
Delivery Methods
Developing safe and efficient systems to deliver HMGA1 or its genetic material specifically to damaged heart tissue.
Regulatory Mechanisms
Understanding the precise signals that control HMGA1 activation and ensuring tight regulation.
Long-term Effects
Assessing the durability of regenerative effects and monitoring for potential late-onset outcomes.
Human Testing
Testing HMGA1 in human heart muscle cells cultured in the lab to assess efficacy and safety.
Collaborative efforts are underway to test HMGA1 in human heart muscle cells cultured in the lab, providing insights into its efficacy and safety in human tissues. These studies will inform the design of clinical trials and the development of gene therapies aimed at reactivating the heart's innate regenerative potential.
Conclusion
The discovery of HMGA1's role in heart regeneration represents a significant advancement in cardiovascular medicine. By elucidating the mechanisms by which this protein loosens chromatin to activate repair genes, researchers have paved the way for innovative therapies that could transform the treatment of heart failure. While challenges remain, the potential to induce heart regeneration in humans offers hope for millions affected by cardiac diseases worldwide.
Works Cited
Hubrecht Institute. "Zebrafish protein unlocks dormant genes for heart repair." ScienceDaily, 2 January 2025. https://www.sciencedaily.com/releases/2025/01/250102162650.htm
Bouwman, M., de Bakker, D., & Bakkers, J. "Cross-species comparison reveals that HMGA1 reduces H3K27me3 levels to promote cardiomyocyte proliferation and cardiac regeneration." Nature Cardiovascular Research, 2 January 2025. https://www.nature.com/articles/s44161-024-00588-9
