Pain in the neuron (HHMI)
Introduction
Every year, millions of people struggle with addiction while seeking relief from chronic pain. In 2022, the CDC estimated that over 82,000 people in the U.S. died from opioid-related overdoses, yet only about 1 in 5 people with opioid addiction received treatment.
In addition, use of opioids like morphine and oxycodone is often accompanied by side effects such as nausea, constipation, sedation. This creates a painful paradox: the very drugs that relieve suffering can also cause addiction and even death.
But what if pain relief didn't have to come with an increasing dependency? What if we could target pain at its source without any adverse side effects?
With the ground-breaking discovery of an experimental drug, SBI-810, Researchers at the Duke University School of Medicine have done just that!
SBI-810 is part of a new generation of drugs designed to target a specific receptor in the nerves and spinal cord — without causing the euphoric "high" typically associated with addiction.
SBI-810 receptor
The Science Explained
SBI-810 is designed to target the brain receptor Neurotensin Receptor 1 (NTSR1). It's responsible for various physiological processes, including the regulation of blood pressure, body temperature, and the response to pain. SBI-810 uses a method called biased agonism to target NTSR1.
The concept of biased agonism (also referred to as functional selectivity) describes how some drugs can selectively activate specific cellular signals while avoiding others, even when acting on the same receptor.
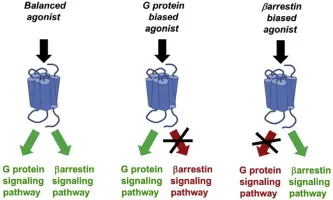
Biased agonism vs. unbiased agonism
Traditionally, scientists believed that when a drug binds to a receptor, it activates all downstream effects equally. In this situation, however, SBI-810 switches on a specific signal — β-arrestin-2 — linked to pain relief while avoiding other signals responsible for side effects or addiction. Another way to describe it would be to "bias" the receptor towards only the beneficial signaling pathway while avoiding the harmful ones.
Compare this:
Traditional opioids, such as morphine, work by binding to special proteins on nerve cells called mu-opioid receptors (MORs). These receptors play a key role in reducing pain, but they're also involved in the brain's reward system and the development of addiction. When a drug like morphine activates these receptors, it triggers two major signaling pathways inside the cell. The first is the β-arrestin-2 pathway, which is responsible for the pain-relieving effects — or analgesia. The second is the G-protein mediated pathway, which leads to many of the drug's unwanted side effects.
These include respiratory depression (slowed or stopped breathing), constipation, increased tolerance (needing more of the drug to get the same effect), and a higher risk of addiction. This dual activation is what makes traditional opioids both effective and dangerous. Biased agonism/functional selectivity suggests that different ligands (molecules binding to a receptor) can induce different changes in a receptor, leading to the activation of specific downstream pathways
In addition to signaling via β-arrestin-2 through NTSR1, SBI-810 doesn't interact with MOR at all, and doesn't activate the G-protein cascades tied to dopamine release. In doing so, SBI-810 contributes to a broader goal: developing effective non-opioid analgesics.

Chemical structure of opioids
Why It Matters
The opioid epidemic continues to plague modern society. Fortunately, in 2024 CDC reported a 27% decrease in deaths due to drug overdose — a hopeful sign. Still, tens of thousands of lives are lost each year, and millions more live with underlying pain. This reflects a deeper issue: some treatments available today often force a trade-off between relief and risk.
That's what makes SBI-810 so significant. Unlike opioids like morphine, it doesn't cause tolerance with repeated use. It also shows potential in real-world scenarios: a study cited in ScienceDaily compared SBI-810 to oliceridine, a newer hospital-based opioid, and found that SBI-810 not only relieved pain effectively but also led to fewer signs of distress in patients.
While it's unlikely to offer an immediate solution to the opioid epidemic, SBI-810 represents a critical step toward long-term change in how we treat pain safely and effectively.
Early Success, But Still Early Days
Despite the potential it holds for the future and for future developments targeted at minimizing the harmful effects of painkillers, SBI-810 remains an experimental drug. In a March 2025 study by Guo et al., it produced strong antinociceptive (reducing detection of a painful stimulus by sensory neurons) effects in mice when administered directly into the spinal cord while also reducing opioid-induced constipation and withdrawal symptoms.
Despite obtaining positive findings, this study shows that SBI-810 has mainly been tested in mouse models, with only a few trials for in vitro systems — many pain drugs that work in animals go on to fail clinical trials. Human dopamine systems are complex, and it's unclear whether similar dopamine-suppressing effects would occur in the same way. The truth is, we're still asking how far a single signaling pathway can take us in the quest to separate relief from risk.
Although SBI-810's future remains uncertain, its underlying approach is a departure from a one-size-fits-all pain medication. It's a signal that the science behind pain and how we treat it is becoming defined by not only what we can block, but what we can leave untouched.
Works Cited
Kenakin, Terry. "Functional Selectivity and Classical Concepts of Quantitative Pharmacology." Pharmacological Reviews, 2011, https://pmc.ncbi.nlm.nih.gov/articles/PMC2948287/.
National Institute on Drug Abuse. "Only 1 in 5 US Adults with Opioid Use Disorder Received Medications to Treat It in 2021." NIDA, 1 Aug. 2023, https://nida.nih.gov/news-events/news-releases/2023/08/only-1-in-5-us-adults-with-opioid-use-disorder-received-medications-to-treat-it-in-2021.
"Neurotensin Receptor 1 Biased Ligand Attenuates Neurotensin-Mediated Excitation of Ventral Tegmental Area Dopamine Neurons and Dopamine Release in the Nucleus Accumbens." UC San Diego Neurosciences, https://neurosciences.ucsd.edu/research/labs/hnasko/_files/Neurotensin-receptor-1-biased-ligand-attenuates-neurotensin-mediated-excitation-of-ventral-tegmental-area-dopamine-neurons-and-dopamine-release-in-the-nucleus-accumbens.pdf.
Centers for Disease Control and Prevention. "Provisional Drug Overdose Death Counts." National Center for Health Statistics, 14 May 2025, https://www.cdc.gov/nchs/pressroom/nchs_press_releases/2025/20250514.htm.
Guo, et al. "Arrestin-Biased Allosteric Modulator of Neurotensin Receptor 1 Alleviates Acute and Chronic Pain." Cell, vol. 186, no. 11, 2025, pp. [page numbers], https://www.sciencedirect.com/science/article/abs/pii/S0092867425005082.
"Experimental Painkiller Could Outsmart Opioids without the High." ScienceDaily, 19 May 2025, https://www.sciencedaily.com/releases/2025/05/250519131126.htm.
